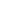Membrane filtration and separation principles
Permeate
Permeation is a common natural phenomenon that occurs when two solutions with different concentrations are separated by a semi permeable membrane. In the process of natural infiltration, water passes through the membrane from the side with lower ion content and enters the solution side with higher ion content. This process will continue until the ion concentration on both sides of the semi permeable membrane is balanced; Or until the pressure difference formed by water penetrating the membrane is equal to the osmotic pressure of the solution.

Reverse osmosis
Reverse osmosis is the process of using sufficient pressure to separate water from a solution through a reverse osmosis membrane, which is opposite to the direction of natural osmosis, hence the name reverse osmosis. According to the different osmotic pressures of various materials, reverse osmosis methods greater than osmotic pressures can be used to achieve separation, extraction, purification, concentration, and other purposes.
If a pressure greater than osmotic pressure is applied to one side of the high concentration solution, the water in the high concentration solution will pass through the osmotic membrane in the opposite direction under pressure and enter the low concentration side, leaving behind ions and suspended solid substances. During the reverse osmosis process, the water passing through the membrane is usually referred to as produced water, and the water left on the other side of the membrane along with dissolved and suspended solids is referred to as concentrated water, saline water, or wastewater.
Osmotic pressure
During the process of natural penetration of a solution, the liquid level on the concentrated solution side continuously increases, while the liquid level on the dilute solution side correspondingly decreases until the water column pressure formed on both sides offsets the migration of solvent molecules. The liquid level on both sides of the solution no longer changes, and the penetration process reaches an equilibrium point. At this time, the liquid column height difference is called the penetration of the concentrated solution
Previous Page:
Related News
undefined